In order to boost up test capacity of SARS-CoV-2 pcr test, products should have reduced the running time while maintaining the analytical sensitivity of existing reagents. STANDARD M SARS-CoV-2 Real-Time Detection kit is a real-time RT-PCR assay intended for the in vitro qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) RNA in human nasopharyngeal swab and oropharyngeal swab specimens. This provides highly sensitive and specific results with shorter running time, which is optimal product for on-site testing at large-scale facilities.
Intented Use
Multiplex real-time RT-PCR test for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper specimens collected from individuals suspected of COVID-19.
Advantage
- Short TAT time (43 minutes of PCR running time)
- Endogenous internal control
- One-Step, One-Tube RT-PCR assay
- High analytical sensitivity
- Optimized primer/probe design for the SARS-CoV-2 detection – ORF1ab gene, N gene
- Contamination prevention system
- Specification
- SpecimenNasopharyngeal swab, Oropharyngeal swab
- Test time43 minutes
- Specimen volume10 µl
- Analytical sensitivity (LoD)ORF1ab (RdRp) gene = 1.0 copies/㎕, N gene = 0.5 copies/㎕
- Compatible systemCFX96
- Storage temperature-25~-15℃ (-13~5 °F)
- Ordering Information
Cat. No. Product Storage temperature Test/Kit 11NCO30 STANDARD™ M SARS-CoV-2 Real-Time Detection Kit -25~-15℃ (-13~5°F) 100 Tests/kit
You must be logged in to post a review.
Vendor Information
- Address:
- No ratings found yet!
-65%
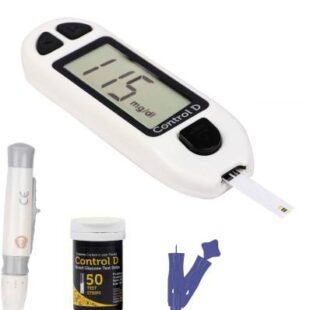
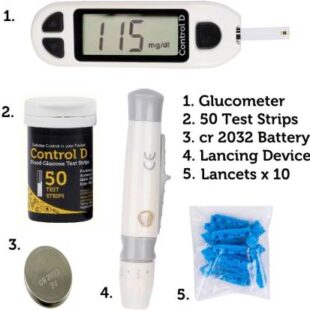
Control D 50 Strips & Automatic Glucose Blood Sugar Testing Glucometer (White)
₹529.00Current price is: ₹529.00. Original price was: ₹1,498.00.
-19%
Abbott Freestyle Flash Glucose Monitoring System, For Clinic, 14 Days
₹4,551.00Current price is: ₹4,551.00. Original price was: ₹5,615.00.
-4%













Reviews
There are no reviews yet.