-
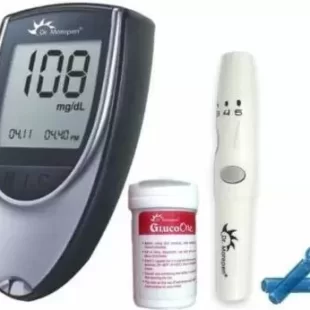
Control D Digital Diabetes Monitor Advanced Glucose Blood Sugar Testing with 25 Strips Glucometer (White, Green)
- Vendor:
Matrics Solution
₹399.00
1
-
Control D Digital Diabetes Monitor Advanced Glucose Blood Sugar Testing with 25 Strips Glucometer (White, Green)
- Vendor:
Matrics Solution
₹399.00














Reviews
There are no reviews yet.